 |
Medical device |
 |
Serial number |
 |
Catalogue reference |
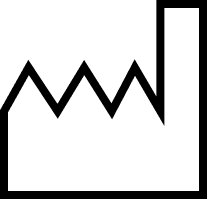 |
Manufacturing date |
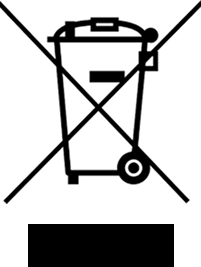 |
Do not dispose of the device with regular household waste. Use the proper disposal system for electrical and electronic waste according to your country's legislation (WEEE) |
 |
Manufacturer |
 |
CE mark |
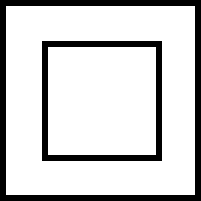 |
Double insulation – battery charger |
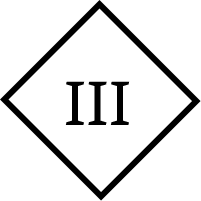 |
Class III – device |
 |
The medical device complies with the requirements of Directive 2011/65/EU and Delegated Directive 2015/863 |
 |
Mandatory reading of the user and maintenance manual |
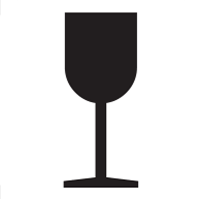 |
Fragile |
 |
Protect the medical device from moisture and weather conditions |
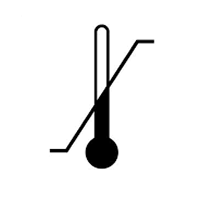 |
5-35°C Storage and operating temperatures |
 |
Useful advice to help the user comfortably operate the device |
 |
Mandatory action |
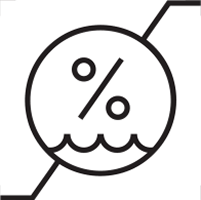 |
≤ 80% non-condensing – Storage and operating humidity |
 |
Non-ionizing radiation connection |
 |
Do not use the medical device if the packaging is damaged |


















